|
||
The following is a very economical way to power these cameras at 1.4 volts, close enough to the original spec to give good meter readings. *** NOTE *** Some cameras (like the Yashica Mat 124G) have a contact at the edge of the battery box for the + connection, rather than using the cap as a contact. If you have one of these, instead of the 18AWG wire indicated in the sketch, use a single-strand 12AWG wire and strip off the insulation. When you roll the wire into a circle, make it slightly undersize so it will fit snugly on the battery and slip it over the battery instead of into the battery box. This will provide the electrical connection to the + contact as well as making the battery fit the box. - Many thanks to Peter Calafell for this tip One or the other of these approaches should take care of almost any camera designed for PX625 mercury batteries. One exception that I know of is the Canonet QL17, which loads the battery edgewise... you'll need to stuff in a little aluminum foil or something to increase the thickness of the 675 battery to make it work in that one. If you're brave enough, you can make an adapter from the shell of a dead 625A alkaline cell: drill a hole in the (-) side near one edge, use a long nose plier to rip that electrode out of the shell. The elecrolyte is a dry gray solid that you will need to clean out of the shell and dispose of. The (+) side of the shell will be your adapter; drop a ZA 675 cell into it and insert it in the camera. If it's too thin, punch a few dimples inward from the (+) side of the shell to raise the 675 cell slightly off the floor and increase the overall thickness for a better fit. 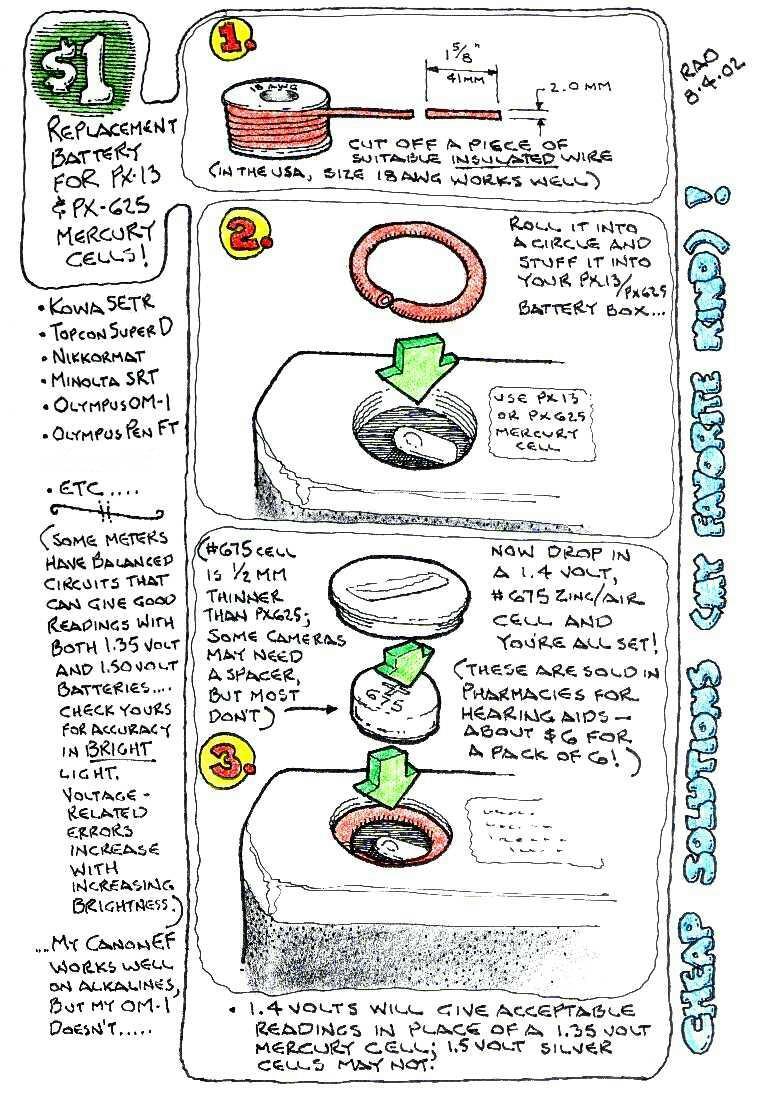
For those who are curious, below is a set of discharge curves for all of the common camera battery chemistry systems. As you can see, mercury and zinc/air essentially share the same curve, while silver has a similar profile but at a higher voltage and alkaline just sort of wanders all over. Of the two lithium curves at the top, please note that the chemistry used in cameras is Li/MnO2, not Li/SO2. The lithium curve does not look as flat as the silver, zinc and mercury cells on this chart, though it's better than alkaline. This data is from a 1986 Duracell publication. [There is another Lithium chemistry, used (I think) only in AA cells, which produces a very flat 1.5V output curve... almost like a silver cell. This is Lithium-Iron Sulfide, or LiFeS.] 
One last bit: This page had originally included links to several commercially available alternatives to the above advice. Over the years since I posted it, these have all become dead links one after another. As time continues to pass, you will be increasingly on your own to find solutions; the advice on this page at least will not expire, as long as size 675 Zinc/Air cells remain available in stores. |
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
|
||
|
